Background
Two commercially available anti-BCMA chimeric antigen receptor T-cell therapies (CAR-T) are
approved by FDA for use in patients with relapsed multiple myeloma (RRMM). Lymphodepletion
(LD) with fludarabine in combination with cyclophosphamide (Flu/Cy) is used as a standard
regimen sequentially prior to CAR-T. Due to a national shortage with regards to availability of
fludarabine, bendamustine (B) has emerged as a possible alternative regimen for LD.
We evaluated the impact of bendamustine as an alternative lymphodepleting regimen to Flu/Cy
on safety and efficacy profile of CAR-T therapy in patients with relapsed multiple myeloma.
Patients and methods
We conducted a single center, retrospective, IRB-approved study for patients with RRMM who
received LD with B and FC at Hackensack University Medical Center (HUMC). Efficacy
outcomes included overall response rates (ORR), Progression free survival (PFS) and Overall
survival (OS). Response to CAR-T was assessed by the treating physician per International
Myeloma Working Group (IMWG) criteria. Safety outcomes included the incidence and severity
of adverse events (AEs). AEs were graded using National Cancer Institute Common
Terminology Criteria for Adverse Events version 5.0 (NCI-CTCAE 5.0). Cytokine release
syndrome (CRS) and immune effector associated neurotoxicity syndrome (ICANS) were graded
as per the American Society for Transplantation and Cellular Therapy (ASTCT) criteria.
Results
We identified 53 patients with RRMM who received anti-BCMA CAR-T therapy between 6/2021
and 6/2023, who had a follow-up of at least 30 days following infusion. Due to shortage of
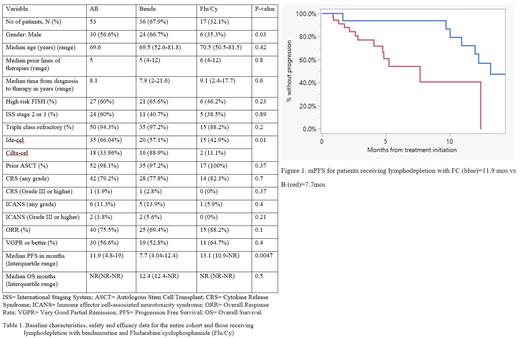
fludarabine, the majority of pts (67.9%) received LD with B. Baseline characteristics are
described in table 1. The median age of the entire cohort was 69.6 years, not significantly
different between the B vs FC group. There were no statistically significant differences between
the two groups with respect to the baseline characteristics except more pts were male in the B
group (66.7% vs 32.1%, p=0.03) and the type of CAR-T product received. No significant
differences were found with regards to the safety profile; however, the ORR were found to be
higher in the FC group compared to the B group (88.2% vs 69.4%) although not statistically
significant (p=0.1). Median PFS (mPFS) was shorted in the B group compared with the FC
group (7.7 months vs 13.1 months) respectively (p=0.0047). Median OS (mOS) was not
reached in the FC group, whereas it was 12.4 months in the B group, p=0.5.
Conclusions
LD with B was not found to have any significant impact on the CRS or ICANS, however a lower
ORR (although not statistically significant) was found. mPFS was shorter for the B group
compared to the FC group which was a statistically significant finding. Although the follow-up is
short, our experience suggests that the use of B as LD may have a negative impact on
outcomes of pts receiving CAR-T therapy for RRMM.
Disclosures
Biran:Pfizer: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Genomic Testing Cooperative: Divested equity in a private or publicly-traded company in the past 24 months; Abbvie: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer Ingelheim: Other: spouse of employee. Suh:Kite Pharma: Membership on an entity's Board of Directors or advisory committees. Siegel:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celularity Scientific: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Parmar:Sanofi: Consultancy, Honoraria; Cellectar Biosciences: Consultancy, Honoraria.